Your shopping cart is empty!
Recombinant Ves v 1 (RP-VV1-1)
| Allergen: | rVes v 1.0101 (Vespula vulgaris allergen 1.0101), Yellow jacket ,wasp |
| Unit: | 250µg |
| Source: | Pichia pastoris |
| Mol. Wt: | 35 kD with dimer 70 kD. |
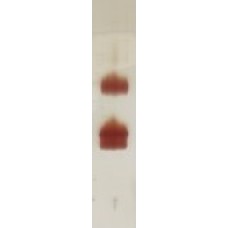
| Purification: | Purified from Pichia pastoris culture by multi-step chromatography. Purity >95% by SDS-PAGE. |
| Concentration: |
See product insert. |
| Formulation: |
Preservative-free and carrier-free in phosphate buffered saline, pH 7.4. Filtered through 0.22µm filter. |
| Storage: | Store at -20ºC. Avoid repeated freeze-thaw cycles. |
| Notes: |
(1) rVes v 1 has a N-terminal 6xHis-tag (2) rVes v 1 has a mutation (H229A) in the active site. (3) rVes v 1 appears as 35 kD monomer and 70 kD dimer on SDS-PAGE. |
| Product Resources: | |
| Allergens are provided for research and commercial use in vitro: not for human in vivo or therapeutic use. | |
| References: | |
| Ollert M, Blank S. Anaphylaxis to Insect Venom Allergens: Role of Molecular Diagnostics. Curr Allergy Asthma. 2015, 15:26. | |
| Mȕller U, Schmid-Grendelmeier P, Hausmann O, Helbling A. IgE to Recombinant Allergens Api m 1, Ves v 1, and Ves v 5 Distinguish Double Sensitization from Crossreaction in Venom Allergy. Allergy. 2012 Aug;67(8):1069-73. | |
|
Seismann H, Blank S, Cifuentes L, Braren I, Bredehorst R, Gunwald T et al. Recombinant Phospholipase A1 (Ves v 1) from Yellow Jacket Venom for Improved Diagnosis of Hymenoptera Venom Hypersensitivity. Clin Mol Allergy 2010:8:7. Monsalve RI, Vega A, Marques L, Miranda A, Fernandez J, Soriano V et al. Component-resolved Diagnosis of Vespid Venom-allergic Individuals: Phospholipases and Antigen 5S are Necessary to Identify Vespula or Polistes Sensitization. Allergy 2012: 67: 528-536. |
|
- Product Code: RP-VV1-1
- Availability: In Stock
-
$665.00